Investigating the role of fibroblast activation in atrial fibrillation pathophysiology
Principal investigators: Simon Haugaard, Sarah Dalgas Nissen, Sofie Kjeldsen, Melodie Schneider, Charlotte Hopster-Iversen and Rikke Buhl
Atrial fibrillation (AF) is a cardiac arrhythmia characterized by chaotic electrical impulses in the atria and is affecting both humans and horses. A better understanding of the pathogenesis of AF is critical in order to improve treatment and prevention of the disease. Atrial fibrillation is a progressive disease that initially is characterized by short and self-terminating episodes. Over time, the episodes become longer and develop into more persistent forms of AF, which are harder to treat. Structural remodeling including fibrotic (scar tissue) formation in the atria may predispose to AF but also develops as a consequence of AF. Preventing this formation may be promising for more successful treatment in the future.
However, our knowledge on the development and role of fibrosis in AF still remains very limited.
Key players in fibrosis formation are the highly abundant fibroblasts, which in the normal heart make up to 25 % of the total cell population. During pathological insults such as AF, fibroblasts are subjected to both mechanical and chemical stimulation, which may lead to fibroblast activation and fibrotic formation. Several studies have shown that the multifunctional enzyme adenosine monophosphate-activated protein kinase (AMPK) serves as an important regulator of this differentiation and if activated this can lower fibroblast activation. Metformin is an antidiabetic drug which is known to activate AMPK and thus serve as a potential new drug in the treatment of structural remodeling in AF patients.
We designed a longitudinal study exploring the anti-fibrotic potential of Metformin in horses with induced persistent AF. In this study, we will follow 20 horses with persistent AF for four month. Half of the horses will be treated with metformin while the others serve as control horses. During the four month, we will benefit from the horses’ very large hearts and collect biopsies continuously from their right atrium. This can be done in horses while they are awake, and only requires a small puncture in one of their large veins in the neck, to gain access to the heart. Figure 1 visualizes the bioptome in the right atrium by echocardiography and the small biopsy is shown in the image to the right. The biopsies will be used to obtain a profound and unique assessment of cardiac tissue properties and to assess the role of fibroblasts in atrial structural remodeling over time during AF. Hopefully we will be able to answer the following questions:
- Does AF lead to activation of fibroblasts in the atrial myocardium, causing increased production of fibrosis? Can we identify a critical time point for this activation?
- Can metformin, by activation of AMPK, inhibit the activation of fibroblasts and differentiation into myofibroblasts and subsequently prevent fibrosis formation? As a result, will the heart then become less prone to AF and the disease burden significantly reduced?
- Can the expression of fibrotic and inflammatory genes that are regulated during AF be correlated to changes in circulating markers in the blood?
The study will be conducted at the Large Animal Teaching Hospital in Taastrup, Denmark, lead by Professor Rikke Buhl. The study comprises a large range of interdisciplinary collaboration involving prominent researchers and facilities from Canada, Germany, Switzerland and Denmark.
The project is funded by the Independent Research Fund Denmark and the Danish Cardiovascular Academy.
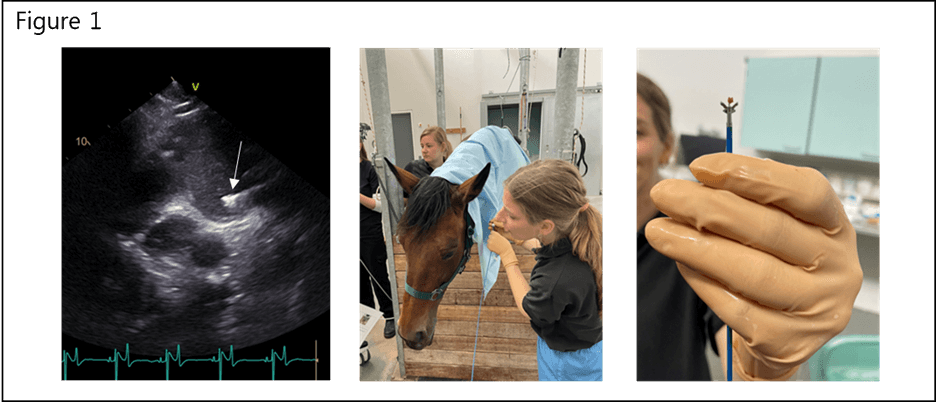
Figure 1. Transvenous biopsy collection. Left: Echocardiography, during biopsy collection, with a right view optimized for visualization of the intervenous tubercle from where the biopsies are harvested. The bioptome is visible in the right atrium (arrow). Middle: The horses are slightly sedated during biopsy collection. Right: The biopsies weights about 5 mg on average and are preserved for extensive gene and protein expression analysis.
