Quantification of contractile and structural remodelling in an equine model of persistent AF – in vivo ultrasound examination and post mortem immunohistochemistry
Principal Investigators: Rikke Buhl and Thomas Jespersen
Study Director: Merle Friederike Fenner
Background:
Unlike electrical and contractile remodelling, structural changes in atrial tissue due to the progression of AF have only been sparsely studied in horses. Therefore, this study focused on quantification of contractile and structural cardiac remodelling in horses developing persistent AF by longitudinal in vivo echocardiography examination and post mortem in vitro immunohistochemistry analysis of cardiac tissue samples.
Aim:
The aim of this study was to quantify the presence of contractile and structural remodelling following the equine model of persistent AF over the course of the disease. Confirmation of previous reported atrial contractile impairment in vivo and investigation of potential differences in fibrosis percentage, fibroblast presence and capillary density between SR and AF tissue specimens in vitro were main points of interest.
 Methods:
Methods:
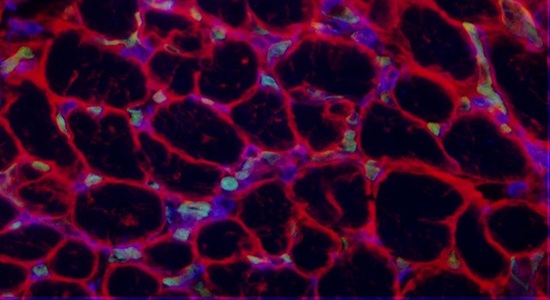
To accomplish this, a standardised echocardiographic protocol as well as a recently developed immunofluorescence triple staining protocol were employed. Tissue sampling for the AF group took place at the Large Animal Teaching Hospital, University of Copenhagen, Taastrup, subsequent to terminal experiments conducted as part of a subsequent study, whereas the SR specimens were retrieved from a Danish horse slaughterhouse. The in vitro validation of the recently for human tissue developed triple-immunofluorescence staining protocol, as well as analysis of resulting data output was carried out in collaboration with researchers from the Cardiovascular Research Institute Maastricht.
Key results:
- Longitudinal progressive left atrial mechanical impairment and the phenomenon of “atrial stunning” subsequent to cardioversion was confirmed.
- GS-IB4 immunofluorescence staining revealed significant capillary rarefaction in equine AF tissue specimens.
- Anti-vimentin immunofluorescence co-staining revealed significantly higher fibroblast density in equine AF tissue samples.
Funding:
This project has received funding from the European Union’s Horizon 2020 MSCA ITN under Grant Agreement No. 675351.


